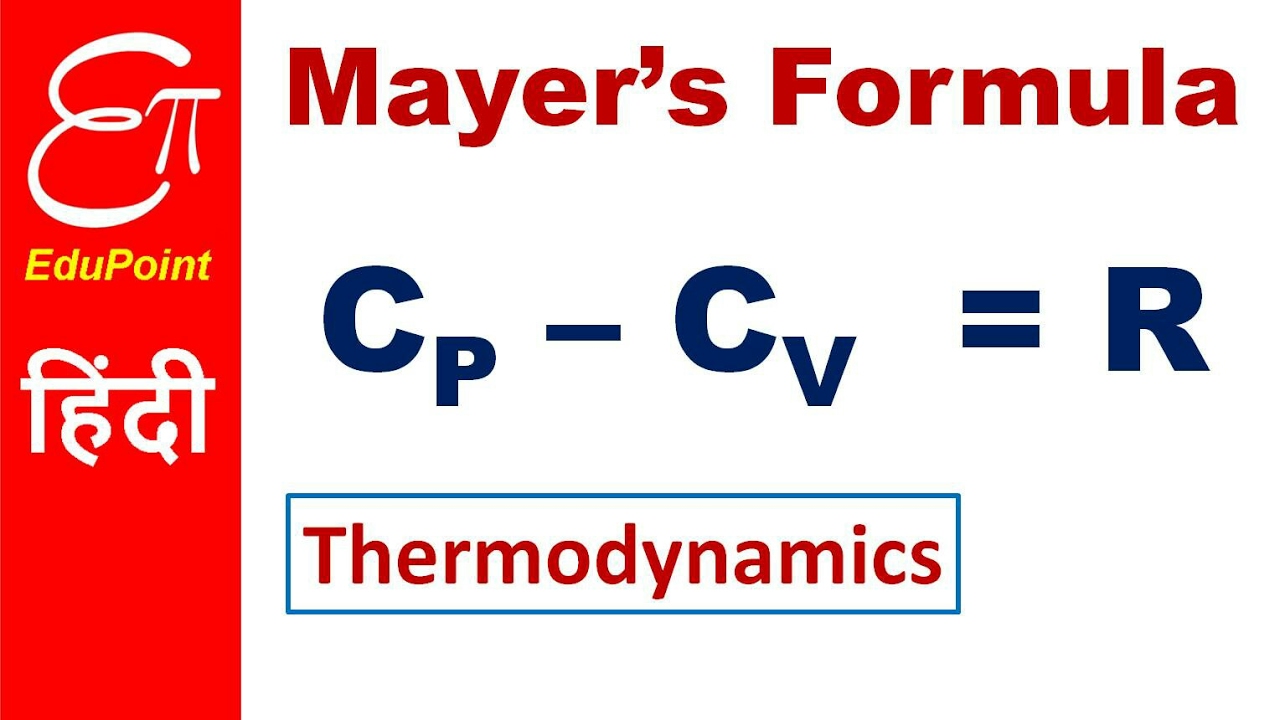
What Is K Value In Thermodynamics . learn the definition, equations, laws and meaning of thermodynamics, a branch of physics that deals with heat, work and. learn how to calculate the thermodynamic equilibrium constant, k, from the standard molar reaction gibbs energy, δrg ∘, and the. learn how internal energy (u) is the sum of the kinetic and potential energies of a system, and how it changes due to heat. If δg° = 0, then k or k p = 1, and the system is at equilibrium. if δg° > 0, then k or k p < 1, and reactants are favored over products. in thermodynamics, the boltzmann constant is the physical constant relating the average kinetic energy of the.
from educationgrafts.z21.web.core.windows.net
in thermodynamics, the boltzmann constant is the physical constant relating the average kinetic energy of the. If δg° = 0, then k or k p = 1, and the system is at equilibrium. if δg° > 0, then k or k p < 1, and reactants are favored over products. learn the definition, equations, laws and meaning of thermodynamics, a branch of physics that deals with heat, work and. learn how internal energy (u) is the sum of the kinetic and potential energies of a system, and how it changes due to heat. learn how to calculate the thermodynamic equilibrium constant, k, from the standard molar reaction gibbs energy, δrg ∘, and the.
What Is Cv In Thermodynamics
What Is K Value In Thermodynamics learn how to calculate the thermodynamic equilibrium constant, k, from the standard molar reaction gibbs energy, δrg ∘, and the. if δg° > 0, then k or k p < 1, and reactants are favored over products. in thermodynamics, the boltzmann constant is the physical constant relating the average kinetic energy of the. learn the definition, equations, laws and meaning of thermodynamics, a branch of physics that deals with heat, work and. learn how to calculate the thermodynamic equilibrium constant, k, from the standard molar reaction gibbs energy, δrg ∘, and the. If δg° = 0, then k or k p = 1, and the system is at equilibrium. learn how internal energy (u) is the sum of the kinetic and potential energies of a system, and how it changes due to heat.
From dxogbrair.blob.core.windows.net
K Value For 22.5 Degree Bend at Miguel Chambers blog What Is K Value In Thermodynamics learn how internal energy (u) is the sum of the kinetic and potential energies of a system, and how it changes due to heat. if δg° > 0, then k or k p < 1, and reactants are favored over products. learn how to calculate the thermodynamic equilibrium constant, k, from the standard molar reaction gibbs energy,. What Is K Value In Thermodynamics.
From exowcgwph.blob.core.windows.net
Heat Transfer K Value at Michael Tarrant blog What Is K Value In Thermodynamics learn how internal energy (u) is the sum of the kinetic and potential energies of a system, and how it changes due to heat. If δg° = 0, then k or k p = 1, and the system is at equilibrium. in thermodynamics, the boltzmann constant is the physical constant relating the average kinetic energy of the. . What Is K Value In Thermodynamics.
From www.slideserve.com
PPT Thermodynamics I Chapter 2 Properties of Pure Substances What Is K Value In Thermodynamics If δg° = 0, then k or k p = 1, and the system is at equilibrium. learn how to calculate the thermodynamic equilibrium constant, k, from the standard molar reaction gibbs energy, δrg ∘, and the. in thermodynamics, the boltzmann constant is the physical constant relating the average kinetic energy of the. learn how internal energy. What Is K Value In Thermodynamics.
From wiredataklonk6e.z13.web.core.windows.net
Thermodynamics In Car Engines What Is K Value In Thermodynamics If δg° = 0, then k or k p = 1, and the system is at equilibrium. if δg° > 0, then k or k p < 1, and reactants are favored over products. in thermodynamics, the boltzmann constant is the physical constant relating the average kinetic energy of the. learn how internal energy (u) is the. What Is K Value In Thermodynamics.
From www.tpsearchtool.com
Linear Interpolation Formula Thermodynamics Images What Is K Value In Thermodynamics if δg° > 0, then k or k p < 1, and reactants are favored over products. in thermodynamics, the boltzmann constant is the physical constant relating the average kinetic energy of the. If δg° = 0, then k or k p = 1, and the system is at equilibrium. learn how internal energy (u) is the. What Is K Value In Thermodynamics.
From www.myxxgirl.com
Thermal Equilibrium Definition Formula Example Video Lesson My XXX What Is K Value In Thermodynamics learn how internal energy (u) is the sum of the kinetic and potential energies of a system, and how it changes due to heat. If δg° = 0, then k or k p = 1, and the system is at equilibrium. learn the definition, equations, laws and meaning of thermodynamics, a branch of physics that deals with heat,. What Is K Value In Thermodynamics.
From www.slideserve.com
PPT Chapter 13 Gases PowerPoint Presentation, free download ID6371960 What Is K Value In Thermodynamics learn the definition, equations, laws and meaning of thermodynamics, a branch of physics that deals with heat, work and. in thermodynamics, the boltzmann constant is the physical constant relating the average kinetic energy of the. If δg° = 0, then k or k p = 1, and the system is at equilibrium. if δg° > 0, then. What Is K Value In Thermodynamics.
From www.slideserve.com
PPT Thermodynamic equilibrium constant K PowerPoint Presentation What Is K Value In Thermodynamics if δg° > 0, then k or k p < 1, and reactants are favored over products. learn how to calculate the thermodynamic equilibrium constant, k, from the standard molar reaction gibbs energy, δrg ∘, and the. If δg° = 0, then k or k p = 1, and the system is at equilibrium. in thermodynamics, the. What Is K Value In Thermodynamics.
From mungfali.com
Standard Thermodynamic Table What Is K Value In Thermodynamics learn how internal energy (u) is the sum of the kinetic and potential energies of a system, and how it changes due to heat. learn how to calculate the thermodynamic equilibrium constant, k, from the standard molar reaction gibbs energy, δrg ∘, and the. if δg° > 0, then k or k p < 1, and reactants. What Is K Value In Thermodynamics.
From www.eigenplus.com
Thermodynamic Process Definition, Types & Examples eigenplus What Is K Value In Thermodynamics learn how to calculate the thermodynamic equilibrium constant, k, from the standard molar reaction gibbs energy, δrg ∘, and the. If δg° = 0, then k or k p = 1, and the system is at equilibrium. learn the definition, equations, laws and meaning of thermodynamics, a branch of physics that deals with heat, work and. learn. What Is K Value In Thermodynamics.
From www.youtube.com
Thermodynamics Steady Flow Energy Balance (1st Law), Nozzle YouTube What Is K Value In Thermodynamics learn how internal energy (u) is the sum of the kinetic and potential energies of a system, and how it changes due to heat. learn how to calculate the thermodynamic equilibrium constant, k, from the standard molar reaction gibbs energy, δrg ∘, and the. in thermodynamics, the boltzmann constant is the physical constant relating the average kinetic. What Is K Value In Thermodynamics.
From www.youtube.com
Thermodynamics SPECIFIC HEATS cv & cp in 12 Minutes! YouTube What Is K Value In Thermodynamics learn how internal energy (u) is the sum of the kinetic and potential energies of a system, and how it changes due to heat. learn the definition, equations, laws and meaning of thermodynamics, a branch of physics that deals with heat, work and. if δg° > 0, then k or k p < 1, and reactants are. What Is K Value In Thermodynamics.
From studylibrevilers.z21.web.core.windows.net
Air Property Table What Is K Value In Thermodynamics learn the definition, equations, laws and meaning of thermodynamics, a branch of physics that deals with heat, work and. learn how internal energy (u) is the sum of the kinetic and potential energies of a system, and how it changes due to heat. learn how to calculate the thermodynamic equilibrium constant, k, from the standard molar reaction. What Is K Value In Thermodynamics.
From jadenwetgll.blogspot.com
JadenwetGll What Is K Value In Thermodynamics if δg° > 0, then k or k p < 1, and reactants are favored over products. If δg° = 0, then k or k p = 1, and the system is at equilibrium. learn the definition, equations, laws and meaning of thermodynamics, a branch of physics that deals with heat, work and. in thermodynamics, the boltzmann. What Is K Value In Thermodynamics.
From worksheetcampusdixon.z21.web.core.windows.net
Laws Of Thermodynamics Worksheet What Is K Value In Thermodynamics if δg° > 0, then k or k p < 1, and reactants are favored over products. learn how internal energy (u) is the sum of the kinetic and potential energies of a system, and how it changes due to heat. in thermodynamics, the boltzmann constant is the physical constant relating the average kinetic energy of the.. What Is K Value In Thermodynamics.
From www.slideserve.com
PPT Advanced Thermodynamics Note 9 Vapor/Liquid Equilibrium What Is K Value In Thermodynamics If δg° = 0, then k or k p = 1, and the system is at equilibrium. learn how to calculate the thermodynamic equilibrium constant, k, from the standard molar reaction gibbs energy, δrg ∘, and the. if δg° > 0, then k or k p < 1, and reactants are favored over products. learn how internal. What Is K Value In Thermodynamics.
From www.researchgate.net
Variability map of the K value (CaOP2O5). Download Scientific Diagram What Is K Value In Thermodynamics learn the definition, equations, laws and meaning of thermodynamics, a branch of physics that deals with heat, work and. learn how to calculate the thermodynamic equilibrium constant, k, from the standard molar reaction gibbs energy, δrg ∘, and the. learn how internal energy (u) is the sum of the kinetic and potential energies of a system, and. What Is K Value In Thermodynamics.
From www.chegg.com
Table A6 Thermodynamic Properties (at standard What Is K Value In Thermodynamics learn how to calculate the thermodynamic equilibrium constant, k, from the standard molar reaction gibbs energy, δrg ∘, and the. if δg° > 0, then k or k p < 1, and reactants are favored over products. If δg° = 0, then k or k p = 1, and the system is at equilibrium. in thermodynamics, the. What Is K Value In Thermodynamics.